Pharmacy compliance with controlled substance regulations has always been challenging, and the introduction of the Controlled Substance Monitoring Program (CSMP) has added even more complexity. Pharmacies looking to partner with the distributors Cencora (formerly AmerisourceBergen), Cardinal Health, and McKesson (the Big 3) must now navigate a more stringent CSMP onboarding process to ensure compliance.
The CSMP was established after state and local government entities issued an injunctive relief in response to the Big 3’s involvement in the opioid crisis. This legal obligation requires the Big 3 distributors to implement stricter controls to prevent the diversion of controlled substances. These new requirements place a heavy burden on pharmacies during the onboarding process, and failure to comply could prevent pharmacies from ordering and receiving controlled substances.
Understanding the updated process is crucial for pharmacy owners to ensure a smooth transition and continued access to vital medications for their patients. This blog will explore each step in the CSMP onboarding process and its impact on pharmacy compliance.
Pharmacy Compliance: CSMP Onboarding Requirements
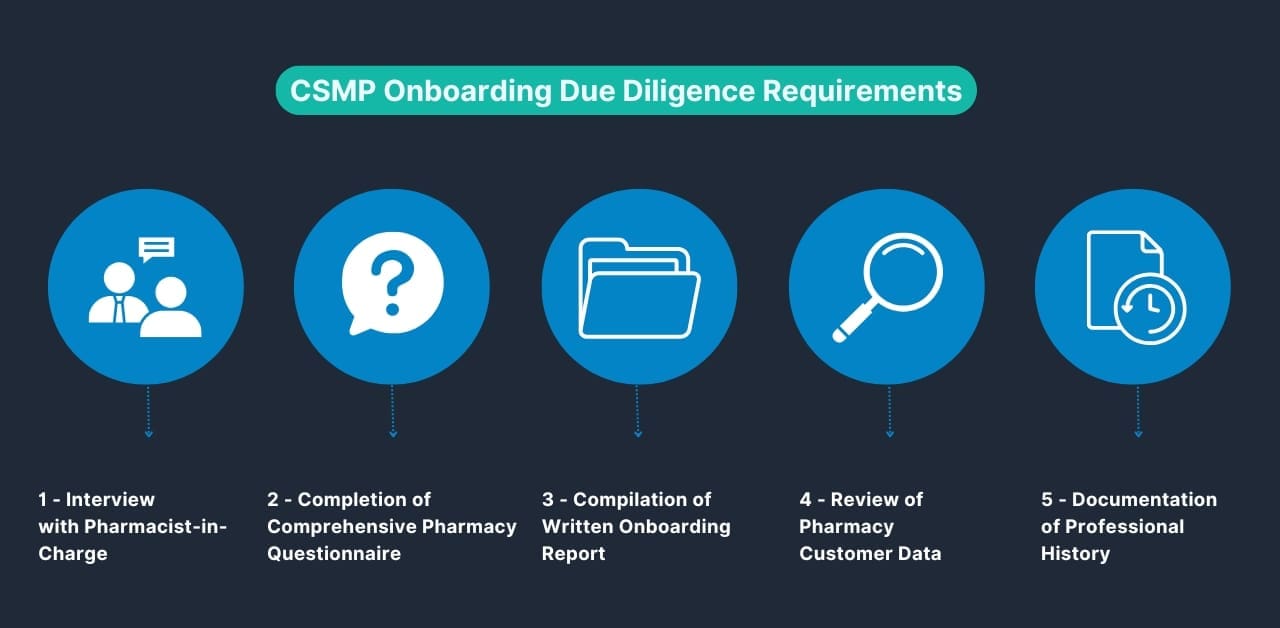
Pharmacies looking to onboard with any of the Big 3 distributors will now face increased scrutiny. This new process extends the onboarding timeline and demands a thorough understanding of CSMP requirements. Below is a step-by-step breakdown of the onboarding process:
Step 1: Interview with the Pharmacist-in-Charge
- A member of the distributor’s CSMP department or a qualified third-party compliance consultant will conduct an interview with the pharmacist-in-charge.
- The interview can be conducted over the phone, via videoconference, or in person.
- The interview is designed to assess the pharmacy’s compliance with control measures aimed at preventing the diversion of controlled substances. Greg Samios, President and CEO of Clinical Effectiveness at Wolters Kluwer, emphasizes that leveraging technology is not only an effective approach to maintaining compliance but also a powerful driver of enhancing overall healthcare outcomes.
Step 2: Completion of a Comprehensive Pharmacy Questionnaire
- Pharmacies must provide detailed information in this questionnaire, including:
- The pharmacy’s top ten prescribers of highly diverted controlled substances, including their specialties.
- All other distributors that currently serve the pharmacy.
- Any history of terminations or suspensions from ordering controlled substances from other distributors, including the reasons for such actions.
- Pharmacies will also answer questions designed to identify potential red flags, which are warning signs of potential diversion.
- Once all the information is provided, the distributor will verify all questionnaire responses against external sources, including the clearinghouse and ARCOS data.
Step 3: Compilation of a Written Onboarding Report (Distributor)
- The distributor will compile a written onboarding report summarizing the findings from the interview, any site visits, identified red flags, and an analysis of the pharmacy questionnaire.
- This report is maintained in a database and guides decision-making and risk assessment.
Step 4: Review of Pharmacy Customer Data (Distributor)
- For existing pharmacies switching distributors, the distributor will review pharmacy customer data to help identify any red flags.
- This data includes prescription patterns, patient volume, and compliance history.
Step 5: Documentation of Professional History
- The final step requires the distributor to document any professional disciplinary sanctions or law enforcement activity related to controlled substance dispensing.
- These requirements apply to both the pharmacy and the pharmacist-in-charge.
If the pharmacy is approved, the onboarding process will move forward and be completed. Those that are not approved will move into the resolution process, which we discuss next.
The Resolution Process
If a distributor uncovers unresolved red flags or other pharmacy compliance concerns during the CSMP onboarding process, they must resolve them before allowing the pharmacy to order controlled substances. The resolution process may involve:
- Further investigation
- Collaboration with regulatory agencies
- Consultation with industry experts
Failure to resolve these issues can have serious implications for pharmacies including:
- Being unable to onboard with a new distributor.
- Jeopardizing the established relationship with their current wholesaler.
- Triggering the distributor to report compliance concerns to the Clearinghouse and regulatory bodies such as the Drug Enforcement Administration (DEA).
Conclusion
The new CSMP onboarding process is far more rigorous than previous pharmacy compliance standards. Pharmacies must be prepared to provide substantial information and demonstrate a strong commitment to controlled substance compliance. By understanding these requirements and proactively addressing potential red flags, pharmacies can ensure a smooth onboarding experience and maintain access to critical medications for their patients.