The Controlled Substance Monitoring Program (CSMP) has significantly changed how distributors manage controlled substances, affecting the way they interact with pharmacies. A crucial aspect of the CSMP is ongoing due diligence, a continuous process that requires both distributors and pharmacies to be extra vigilant when maintaining controlled substance compliance.
The distributors: Cencora (formally AmerisourceBergen), Cardinal Health, and McKesson (the Big 3) are now legally obligated to perform regular and comprehensive audits and reviews of their pharmacies’ controlled substance handling, thanks to a court-imposed injunctive relief. To fulfill their due diligence, the Big 3 each implemented their own CSMP to collect data, analyze trends, and identify potential red flags. Pharmacies that fail to meet their distributors’ CSMP database and controlled substance compliance requirements could face serious consequences, including the inability to order controlled substances. This blog will discuss the key elements of the CSMP’s ongoing due diligence efforts and how pharmacies can still maintain controlled substance compliance.
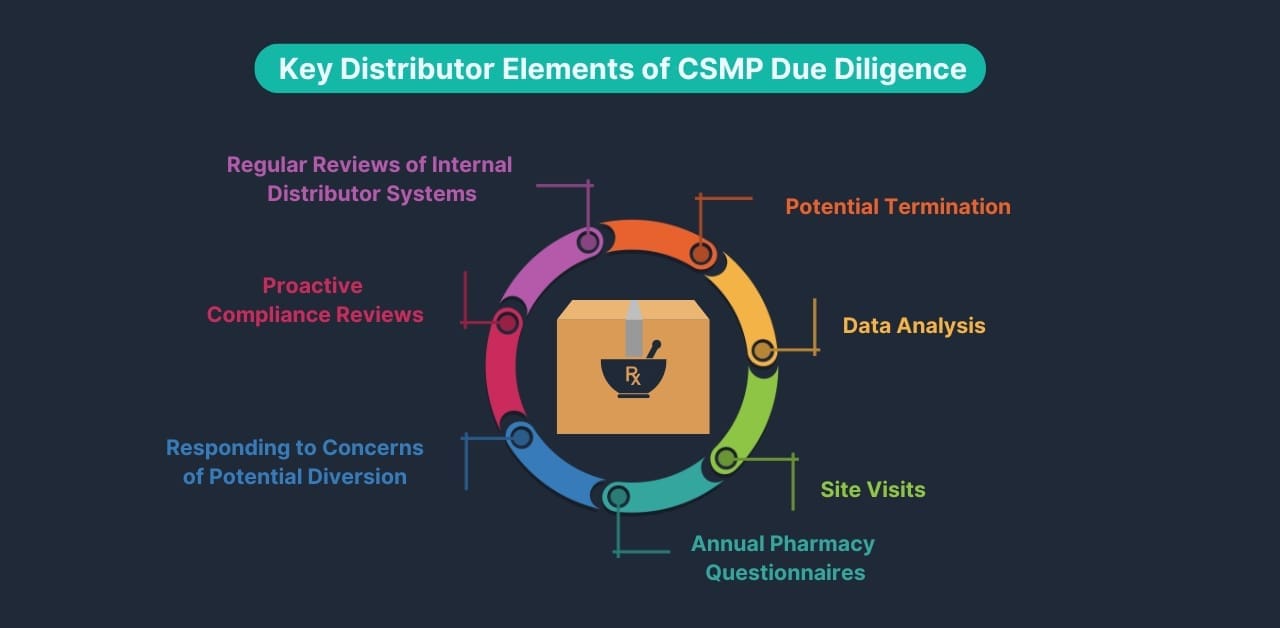
Seven Key Distributor Elements of CSMP Due Diligence
As pharmacists, it is incredibly valuable to understand the impact of ongoing due diligence from the distributor’s perspective. This section outlines seven general CSMP due diligence requirements for distributors, helping pharmacies understand the information their distributors need and how to adjust their workflows to support controlled substance compliance.
1. Regular Reviews of Internal Distributor Systems:
Distributors must proactively review their procedures and systems to ensure they can effectively detect patterns and trends in pharmacy ordering data. This includes utilizing data from the Automated Reports and Consolidated Orders System (ARCOS) provided by the Drug Enforcement Administration (DEA) and the CSMP database (Clearinghouse).
2. Proactive Controlled Substance Compliance Reviews:
Distributors are required to conduct proactive controlled substance compliance reviews of their pharmacy customers. These reviews assess the pharmacies’ adherence to regulations and effectiveness in controlling against diversion.
3. Responding to Concerns of Potential Diversion:
Distributors must conduct thorough investigations when there are concerns about potential diversion at a pharmacy. Triggers for these investigations include:
- Unresolved red flags
- Information from law enforcement or regulators
- Suspension or revocation of a pharmacist’s DEA registration or state license
- Information received through the distributor’s hotline
- Information from other distributors
- Any other reliable information suggesting diversion
4. Annual Pharmacy Questionnaires
Distributors will request updated pharmacy questionnaires annually from 500 of their customers. These questionnaires gather crucial information about:
- Top prescribers of highly diverted controlled substances
- Other distributors serving the pharmacy
- Past terminations or suspensions
- Information relevant to identifying red flags
The top 250 pharmacies by volume of highly diverted controlled substances are automatically included in this annual review. The remaining 250 pharmacies are selected on risk-based criteria developed by the distributor’s Chief Diversion Control Officer (CDO).
5. Site Visits:
Distributors are obligated to conduct site visits, including unannounced visits, as part of their CSMP due diligence requirements. These visits allow distributors to:
- Observe pharmacy operations firsthand
- Interview the pharmacist-in-charge and other staff
- Review compliance documentation
All findings from site visits are documented and maintained in a CSMP database accessible to relevant personnel.
6. Data Analysis:
Under the injunctive relief, distributors must regularly analyze pharmacy customers’ ordering and dispensing of controlled substances. This includes monitoring for any licensing or other regulatory actions involving the customer or its pharmacists. Distributors will be required to collect 90 days of de-identified dispensing data from their pharmacies more frequently.
7. Potential Termination:
Distributors must take decisive action if they determine that a pharmacy poses a significant diversion risk. This could include terminating the pharmacy’s ability to order controlled substances.
CSMP Preparation for Pharmacies is Key
Pharmacies need to be proactive in preparing for CSMP due diligence requirements. Here are five essential steps pharmacies can take to ensure controlled substance compliance:
1. Regular Training and Education:
Provide comprehensive training to all staff members on CSMP due diligence requirements, diversion prevention techniques, and proper record-keeping procedures.
2. Internal Audits and Assessments:
Regularly conduct internal audits to assess controlled substance compliance with all aspects of the CSMP. This includes audits of inventory management, prescription filling, staff training, and record-keeping practices.
3. Robust Compliance Protocols:
Develop and implement strong compliance protocols that address all aspects of controlled substance handling, from ordering and receiving to dispensing and record-keeping.
4. Collaboration with Distributors:
Maintain open communication with your distributor and proactively address any concerns they raise regarding controlled substance compliance. Utilize your distributor as a resource for CSMP due diligence preparation.
5. Stay Informed and Updated:
Continuously monitor industry publications, regulatory updates, and guidance from professional organizations to stay abreast of any changes or clarifications to CSMP due diligence requirements.
Conclusion
By prioritizing controlled substance compliance and adopting a proactive approach, pharmacies can seamlessly navigate CSMP due diligence processes while ensuring continued access to essential medications for their patients. To learn more about the CSMP, explore the other articles in our CSMP Blog Series.