Managing controlled substance compliance is often a polarizing topic among healthcare professionals. While every pharmacy may have its own preferred approach, one requirement remains universal: all DEA-registered dispensers must maintain a complete and accurate biennial inventory report. Unfortunately, these reports are often viewed as a burdensome task or simply another item on the compliance checklist. In reality, they serve as a critical safety measure that protects patients, staff, and the community.
In this blog, we will walk through everything you need to know about biennial inventory reports. We will explain what they are and why they matter, discuss the challenges pharmacies face in maintaining them, and highlight the benefits of making them part of a proactive compliance strategy. Finally, we will share how C2 Keep can simplify the process, reduce stress, and ensure reporting becomes a seamless part of your workflow.
What Is a Biennial Inventory Report and Why Conduct them Regularly?
According to the Drug Enforcement Administration (DEA), biennial inventory reports accounting for all controlled substances on hand are required for each DEA registered business at least once every two years. Each report must include:
- The date of the inventory
- Whether the inventory was taken at the beginning or the end of the day
- The name of each controlled substance inventoried
- The finished form of each substance
- The number of dosage units in each container
- The number of commercial containers of each finished form
- The total count of the substance
Although not required, the DEA also recommends including the registrant’s name, address, DEA registration number, and the signature of the person responsible for taking the inventory.
While the minimum requirement is every two years, conducting biennial inventory reports more frequently provides significant advantages. One of the most important is readiness for unexpected audits. During these audits, a DEA agent or state board representative reviews compliance records and processes to identify vulnerabilities to drug diversion. Having an up-to-date report readily available not only proves compliance but also demonstrates a commitment to going above and beyond in safeguarding patients and communities.
Regular reporting also minimizes the risks associated with noncompliance. Penalties under the Controlled Substances Act (CSA) continue to rise, with each record-keeping violation costing $19,246 and each prescription violation reaching $82,950. Violations can also result in a Corrective Action Plan (CAP) or, in severe cases, loss of DEA registration. More importantly, conducting regular biennial inventory reports ensures that all controlled substances are accounted for, allowing discrepancies to be identified and corrected before they escalate.
Despite these clear benefits, many healthcare professionals still struggle to make biennial inventory reports a consistent part of their workflow.
The Challenge of Conducting Frequent Biennial Inventory Reports
Time management is one of the biggest factors influencing how pharmacies approach compliance. Pharmacies across the country are busier than ever, with compliance representing only one responsibility among many. The recent influx of patient transfers from chains such as CVS, Walgreens, and Rite Aid has further strained workflows, leaving many pharmacies relying on outdated systems that cannot keep pace with today’s demands.
Manual reporting processes are a major part of the problem. Paper logs and spreadsheets are cumbersome, and creating biennial inventory reports often requires hours of transferring data into a properly formatted report. Beyond the time commitment, manual methods introduce greater risk of error. Misreading handwriting, typing in the wrong number, or skipping an entry can all result in costly mistakes that put a pharmacy at risk of noncompliance.
The financial burden is another challenge. In the current era of Pharmacy Benefit Manager (PBM) reform, where prescription margins are already shrinking, dedicating several hours or even a full workday to completing a report translates into hundreds of dollars in labor costs. Over time, these costs accumulate and cut into already thin margins.
The good news is that pharmacies do not have to continue struggling with outdated methods. With the right tools in place, the reporting process can become faster, more accurate, and significantly less costly.
How C2 Keep Makes Biennial Reporting Seamless
At C2 Keep, we understand that pharmacies are stretched thin, especially with growing patient demand and increased compliance requirements. That is why our mission is to remove the stress from controlled substance management and give pharmacists more time to focus on patient care.
Our cloud-based, fully integrated compliance platform is designed to meet DEA requirements under the Controlled Substances Act (CSA) while simplifying every step of the process. Customers consistently tell us how much they appreciate the way C2 Keep streamlines the creation of biennial inventory reports. What often takes an entire workday to complete can be finished in just minutes.
Here’s how C2 Keep makes it possible:
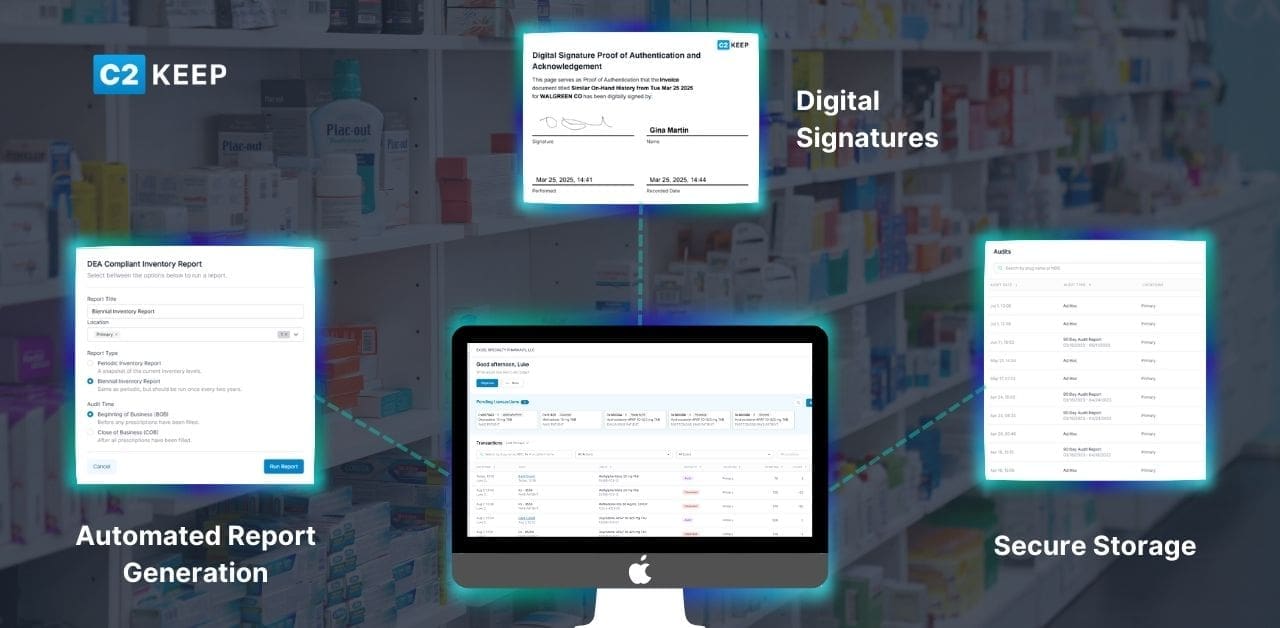
- Automated Report Generation: Biennial inventory reports are created instantly by pulling directly from your existing inventory data into a DEA-compliant template.
- Digital Signatures: Multiple signatures can be securely created, saved, and applied, allowing both the creator and witness to sign digitally.
- Secure Storage: All reports are stored safely within C2 Keep and are always available for download or export.
Running biennial inventory reports regularly is one of the simplest ways to demonstrate compliance and accountability. With C2 Keep, it no longer feels like a burden. That is why more than 1,500 pharmacists, staff members, and healthcare professionals trust C2 Keep as their one-stop solution for controlled substance compliance.
Conclusion
Prioritizing biennial inventory reports is one of the most important steps pharmacies can take to maintain compliance and strengthen patient safety. The pharmacy industry, like much of the healthcare sector, is experiencing rapid technological change. Patients expect more convenience, and pharmacies must adapt to meet these rising expectations. At the same time, the aging baby boomer generation and the increasing demand for long-term care are driving a steady rise in controlled substance prescriptions. Combined with heightened regulatory requirements, this creates growing pressure to improve efficiency while maintaining the highest safety standards.
C2 Keep was designed to meet these challenges head-on. Our platform provides a complete solution for controlled substance compliance, helping pharmacies stay organized, compliant, and audit-ready while freeing up valuable time to focus on patient care.
If you are ready to strengthen compliance and protect your community, schedule a demo with our founder and CEO, Roland Achenjang, to see how C2 Keep can support your pharmacy.
