With so much happening in a healthcare practice each day, it is easy for certain controlled substance compliance requirements to slip through the cracks, especially those involving damaged, expired, or destroyed inventory. While many practitioners place strong emphasis on managing their initial and perpetual inventories, controlled substance disposal activities are sometimes overlooked, despite being just as critical to Drug Enforcement Administration (DEA) compliance.
Disorganized records, manual processes, and inconsistent protocols are often the culprits behind compliance gaps. Even when circumstances are unavoidable, inefficient disposal practices can lead to costly fines, stressful audits, or worse. While compliance risks can never be eliminated entirely, healthcare professionals can take clear steps to minimize exposure.
This blog will outline what every DEA registrant needs to know about proper controlled substance disposal. We will review DEA requirements for destruction, the necessary forms for each type of transaction, and the records that must be kept readily accessible for federal and state officials. We will also highlight best practices to strengthen your compliance processes. Finally, we will show how digital compliance tools like C2 Keep make it easier to document, track, and manage disposal activity with confidence. With the right systems in place, maintaining compliance does not have to feel overwhelming.
DEA Requirements for Controlled Substance Disposal
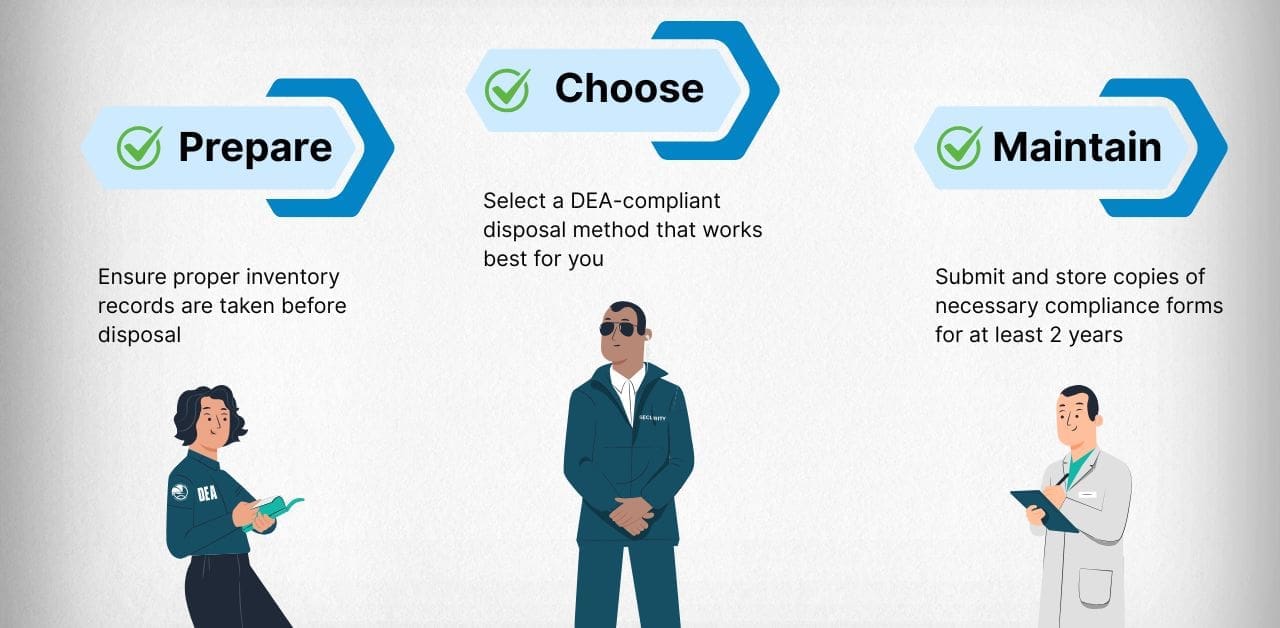
Regardless of the reason for controlled substance disposal, all activity must follow strict DEA guidelines. There are three main areas practitioners need to understand:
Preparing for Disposal
Before starting the process of controlled substance disposal, an inventory record must be created and stored separately for at least two years. Pharmacies typically run audits to identify substances that need destruction. Once identified, the pharmacy is required to separate those substances from the rest of the inventory and create a new record that includes:
- The name of the substance
- The total quantity of the substance to the nearest metric unit weight, or the total number of finished units
- The reason the substance is being maintained by the registrant
- Whether the substance could be used in the manufacture of a finished controlled substance
Choosing a Destruction Method
Once records are complete, the practitioner is responsible for selecting a compliant destruction method. According to the DEA, there are four primary options:
- On-Site Destruction: Practitioners may destroy their own inventory if it complies with all federal, state, tribal, and local regulations and the substances are rendered non-retrievable.
- Reverse Distribution: Practitioners may deliver their controlled substance to a registered reverse distributor, or arrange for pick-up at their location.
- Return to Manufacturer: Substances can be delivered to or picked up by the original manufacturer, the registered distributor, or another registrant authorized to accept returns or recalls.
- Contact Local DEA Agent: A practitioner may request guidance from their local DEA special agent for further instructions.
Maintaining the Right Forms
Proper documentation is essential for compliance. The primary form associated with controlled substance disposal is DEA Form 41, which documents the method of destruction. When Schedule II drugs are transferred to another DEA registrant for destruction, DEA Form 222 must be completed. Pharmacies outsourcing destruction will also receive a Certificate of Destruction (COD) as proof of safe, compliant disposal.
As with all controlled substance records, copies of these forms must be stored and readily accessible for at least two years.
Best Practices for Managing Damaged or Expired Substances
Managing damaged or expired controlled substance can feel overwhelming, but pharmacies can adopt clear, proactive strategies to ensure compliance and maintain safety. By focusing on key areas, pharmacists can reduce errors, prevent diversion, and simplify the audit process.
Prioritize Inventory Transparency
The foundation of proper controlled substance disposal is visibility. Damaged or expired inventory must be tracked transparently so discrepancies do not go unnoticed. A common challenge for pharmacies is siloed workflows, where disconnected processes make it difficult to maintain accuracy. This lack of visibility often results in undetected errors and stressful audits. Maintaining perpetual inventory records and giving authorized staff real-time access helps pharmacies catch issues early and respond appropriately.
Restructure Workflows for Better Organization
Transparency is only effective when supported by organized workflows. Without structure, managing disposal can feel like a game of “telephone,” where each additional step increases the risk of miscalculations, misread handwriting, or misplaced records. Streamlined, integrated workflows reduce these risks. When data flows seamlessly across the pharmacy without unnecessary handoffs, records stay accurate, files remain organized, and compliance tasks become far less burdensome.
Make it a Routine
Controlled substance disposal is not a one-time event but an ongoing responsibility. Pharmacy leaders should establish repeatable processes to ensure safe and compliant management at all times. Regular self-audits confirm that logs are accurate, documentation is complete, and required forms are readily available. Standard Operating Procedures (SOPs) should clearly outline how staff must handle substances identified for destruction, leaving no room for confusion during audits or compliance checks.
How C2 Keep Makes Managing Them a Breeze
Managing damaged and expired inventories does not have to be difficult with C2 Keep. Built with pharmacy expertise at its core, C2 Keep is a cloud-based, fully integrated controlled substance compliance and management solution. As a comprehensive solution for compliance, C2 Keep helps manage controlled substance disposal activities in several key ways:
- Separate Inventory Locations: C2 Keep meets DEA requirements by automatically separating damaged and expired products from the active, dispensable inventory.
- Digital File/Signature Storage: Users can attach and sign DEA 41 forms, distributor invoices, or any other required documents directly within each activity. This keeps files organized and readily accessible during audits.
- Automated Report Generation: Pharmacies can generate DEA and state compliant reports for all damaged and expired items within seconds.
- Complete Audit Trail: Whether a product is sold to a reverse distributor or destroyed, C2 Keep allows users to record a matching transaction that documents the final disposition.
- Secure Records: User-specific access controls help protect sensitive information. Pharmacies can also record a witness for any destruction that occurs locally.
Conclusion
Controlled substance disposal and the management of damaged or expired inventories do not need to weigh down your pharmacy. The real challenge lies in balancing efficiency with effectiveness. The most efficient workflows fail without the right tools, while the most advanced tools lose value without strong, well-structured processes in place.
That’s why C2 Keep was created. After experiencing these challenges firsthand, C2 Keep founder and CEO Roland Achenjang designed a platform that unites compliance, organization, and accountability in one seamless solution. Today, more than 2,000 pharmacists and staff rely on C2 Keep every day to simplify audits, strengthen compliance, and streamline controlled substance disposal.
Ready to see how C2 Keep can help your pharmacy achieve both efficiency and effectiveness? Schedule a demo with Roland today.